Polycyclic Aromatic Hydrocarbons
Polycyclic Aromatic Hydrocarbons
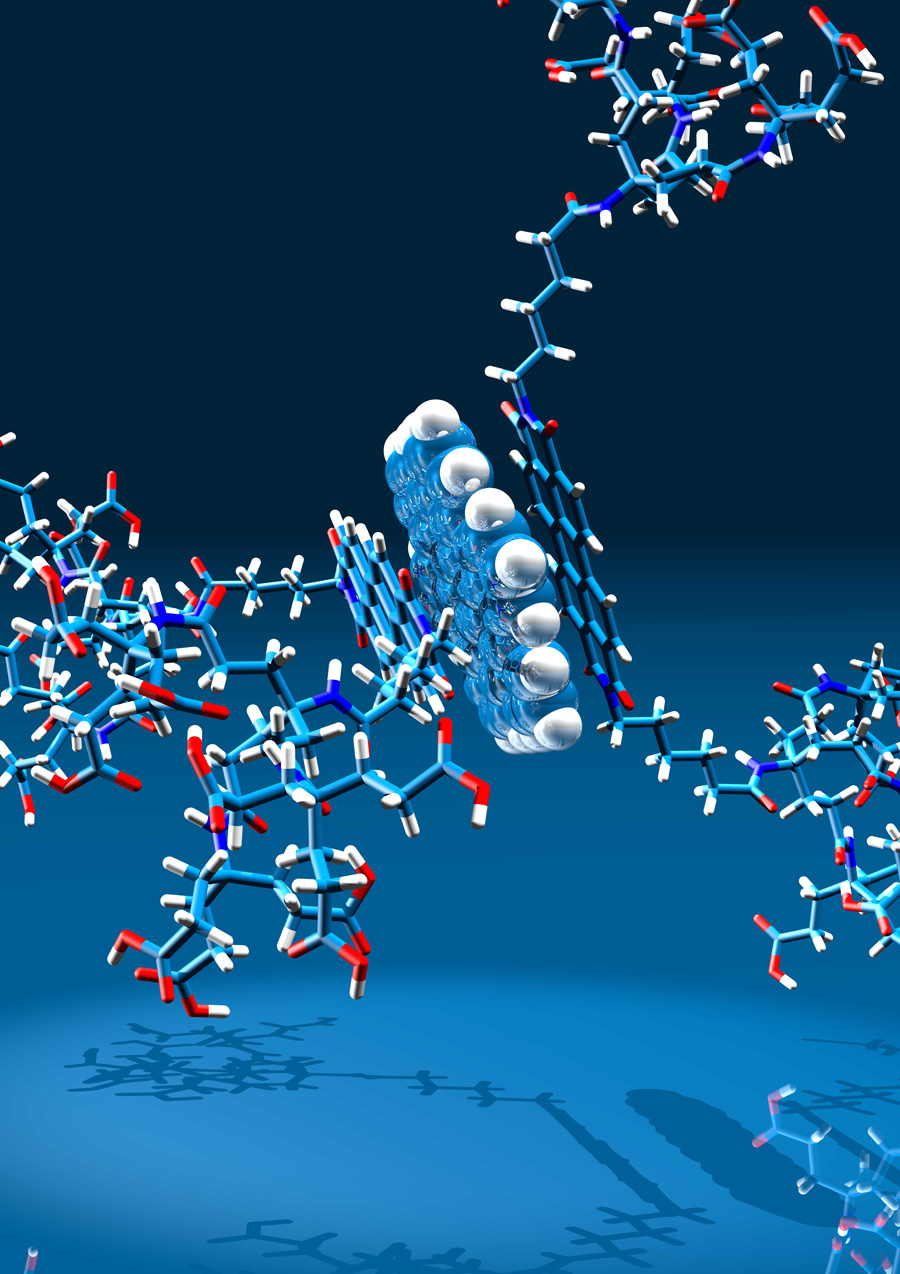
Polycyclic aromatic hydrocarbons such as hexabenzocoronenes and rylenes (naphtylenes, perylenes) do not only represent partial structures of – and model compounds for – graphene; they also represent attractive building blocks for new molecular architectures. We are particularly interested in using them as chromophores, as redox-active components and as structure determining tectons for functional hybrid molecules. Other compound classes that we are extensively investigating with respect to new derivatization concepts are porpyhrine based molecules and calixarenes. Among our accomplishments in the chemistry of all these conjugated π-systems are the first examples of water-soluble perylenes and their use for non-covalent graphene functionalization, dendritic metalloporphyines as heme-proteine models and amphiphilic calixarenes as building blocks for the first shape persistent micelles.
Selected Publications
- , , , , :
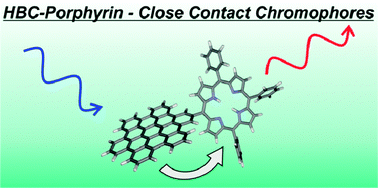
HBC-porphyrin-close contact chromophores
In: Chemical Communications 49 (2013), p. 4827-4829
ISSN: 1359-7345
DOI: 10.1039/c3cc41740a
A photo/redoxactive hexa-peri-hexabenzocoronene-porphyrin conjugate with a direct connection between the two chromophores was synthesised using a formylated hexaphenylbenzene precursor.
The new synthetic carbon allotrope graphene has recently attracted much attention due to its unprecedented physical properties. Since graphene is a zero band-gap semiconductor, proper functionalization and/or modification of its structure is mandatory to open a band-gap, which represents an important prerequisite for many possible applications in the field of molecular electronics. In this regard, covalent attachment of photoactive molecules is a challenging subject. Next to the introduction of a band-gap such a chemical modification would also be associated with the development of interesting photo/redox activity profiles.
Wet chemical functionalization of graphene
In: Accounts of Chemical Research 46 (2013), p. 87-96
ISSN: 0001-4842
DOI: 10.1021/ar300116q
On the basis of our experience with fullerenes and carbon nanotubes, we have described a series of covalent and noncovalent approaches to generate graphene derivatives. Using water-soluble perylene surfactants, we could efficiently exfoliate graphite in water and prepare substantial amounts of single-layer-graphene (SLG) and few-layer-graphene (FLG). At the same time, this approach leads to noncovalent graphene derivatives because it establishes efficient π–π-stacking interactions between graphene and the aromatic perylene chromophors supported by hydrophobic interactions.
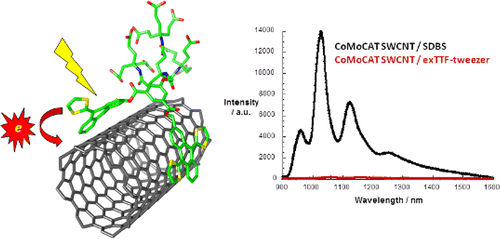
Tetrathiafulvalene-based nanotweezers-noncovalent binding of carbon nanotubes in aqueous media with charge transfer implications
In: Journal of the American Chemical Society 134 (2012), p. 9183-9192
ISSN: 0002-7863
DOI: 10.1021/ja211362z
As a suitable electron donor, π-extended tetrathiafulvalene (exTTF) stands out owing to its recognition of SWCNT through π–π stacking and electron donor–acceptor interactions. Herein, we explore the shape and electronic complementarity between different types of carbon nanotubes (CNT) and a tweezers-shaped molecule endowed with two exTTFs in water. The efficient electronic communication between semiconducting SWCNT/multiwall carbon nanotubes (MWCNT), on one hand, and the water-soluble exTTF nanotweezers 8, on the other hand, has been demonstrated in the ground and excited state by using steady-state as well as time-resolved spectroscopies, which were further complemented by microscopy.
- , , :
The potential of perylene bisimide derivatives for the solubilization of carbon nanotubes and graphene
In: Advanced Materials 23 (2011), p. 2588-2601
ISSN: 0935-9648
DOI: 10.1002/adma.201100300
- , , , , , , , :
Non-covalent chemistry of graphene: Electronic communication with dendronized perylene bisimides
In: Advanced Materials 22 (2010), p. 5483-5487
ISSN: 0935-9648
DOI: 10.1002/adma.201003206
Mutual attraction: π–π bonding promotes electronic interactions between graphene and a π-conjugated perylene in a liquid dispersion. Herein, we report for the first time on the electronic communication between graphene with the perylene bisimide (PBI) when both are deposited on a surface or dispersed in homogeneous solution. This interaction is provided by the non-covalent binding of their conjugated π-systems.
- , , :
Synthesis and aggregation properties of water-soluble newkome-dendronized perylenetetracarboxdiimides
In: European Journal of Organic Chemistry (2007), p. 5497-5505
ISSN: 1434-193X
DOI: 10.1002/ejoc.200700567
The synthesis and characterization of three highly water-soluble perylenetetracarboxdiimide (“perylene bisimide”, PBI) dyes 1 and 2b is presented. The water solubility is provided by peripheral dendronization with 1G- and 2G-Newkome dendrons. The aggregation behaviour of these amphiphiles in water and that of their tert-butyl-protected precursor molecules 3 and 4 in organic solvents was investigated by UV/Vis- and fluorescence spectroscopy. The symmetric 1G-dendronized dye 1a forms aggregates more easily than its 2G-counterpart 1b. The asymmetric derivatives 2b reveals pronounced aggregation properties due to the presence of both a 2G-dendron and a flexible and non-bulky dodecyl substituent. The 1G-analogue 2a is insoluble in water as a result of insufficient overall hydrophilicity caused by only one 1G-Newkome dendron. Within the series of water-soluble perylenes 1 and 2b the asymmetric derivative 2b forms the most regularly shaped micelles in water (pH = 7.2) with a mean diameter of 16 nm as demonstrated by transition electron microscopy (TEM). The bola-amphiphiles 1a and 1b form a distribution of smaller aggregates on average with less defined shape. (© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2007)